Project B06
Synaptic plasticity mechanisms regulating fear memory and fear extinction learning

Prof. Dr. Volkmar Leßmann
To develop an appropriate and adaptive motivated behavior, the ability to form an associative memory of stimuli accompanying positive or negative events is essential. Stimuli occurring before or together with an aversive event are negatively associated and subsequently induce avoidance behavior, and thus fear learning. In our project we analyze the synaptic circuits of the amygdala and the hippocampus that contribute to the formation of fear memories. In particular, we are interested in the function of BDNF (brain-derived neurotrophic factor) and dopamine (DA), which are known to crucially regulate synaptic plasticity in this learning paradigm. Accordingly, we use a combined approach of in vivo behavioral and pharmacological experiments with in vitro / ex vivo field potential and patch clamp recordings to analyze the role of BDNF and DA in fear learning.
In recent years we demonstrated an essential role of BDNF in the acquisition and consolidation of fear memories as well as for long-term potentiation (LTP) in the amygdala network (Fig.1). As the synaptic network of the amygdala is highly modulated by GABAergic interneurons, an additional focus of our work lies on cellular mechanisms of synaptic plasticity of GABAergic circuits. Specifically, we want to gain mechanistic insights into the function of the different types of inhibitory networks in the basolateral amygdala (BLA) and the central amygdala, focusing on somatostatin and PKCδ expressing interneurons.

We analyze spike-timing-dependent plasticity (STDP) – a physiologically important type of LTP – in hippocampal and amygdala slices. Using STDP paradigms in hippocampal brain slices we identified different BDNF-dependent and -independent types of timing-dependent (t-)LTP (Edelmann et al., 2015; Fig.2) that are also relevant for amygdala inputs. We currently investigate the modulation of critical time windows of pre- and postsynaptic activation yielding t-LTP by BDNF and DA. In collaboration with other partners of the CRC 779 we will investigate to which extent local application of these neuromodulators in the hippocampus can affect trace fear learning. Overall, with this combined approach we expect to gain insights into potential mechanisms of timing-relevant processes in associative learning and their modulation.
Since fear memories are very resistant against forgetting, they need to be modulated by additional learning processes, i.e., fear extinction learning, to adapt them to the actual environmental situation. We showed previously that BDNF+/- mice exhibit an age-dependent learning deficit that is accompanied by reduced BDNF expression in learning relevant brain areas (Fig.3). Furthermore, we identified a learning-induced increase in BDNF protein in the medial prefrontal cortex (mPFC), suggesting an involvement of prefrontal BDNF in the consolidation of extinction memories. In the future we aim at pinpointing the impact of BDNF processing on fear extinction learning as well as on long-term depression (LTD) in the BLA. In collaboration with our partners in the CRC we will analyze the specificity of BDNF signaling in the mPFC as well as its role in contextual processing of fear extinction.
In conclusion, by applying a combined approach of in vivo, ex vivo and in vitro techniques, we thus far identified important functions of BDNF in fear and fear extinction learning. In the upcoming funding period we want to focus on GABAergic synaptic mechanisms of the inhibitory networks in the amygdala in fear learning as well as on modifications of timing-related plasticity by neuromodulators. In addition, we will continue to analyze the role of BDNF in fear extinction learning and the contextual processing of extinction memories.
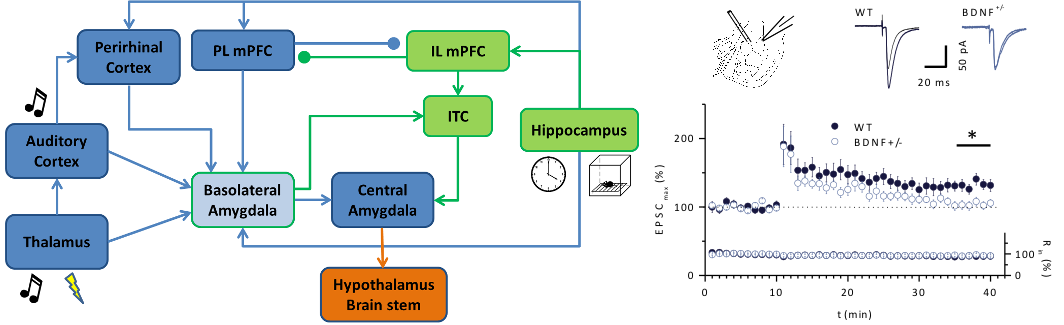
Fig 1: Fear and fear extinction learning in BDNF+/- mice. Left: Neural circuitry of the key brain areas involved in the processing of cued fear learning (blue) and fear extinction learning (green) [modified from Maren (2011) Neuron]. Right: Impaired LTP at the thalamic input of BDNF+/- mice. LTP pairing was induced by pairing afferent stimulation of the thalamic input (100 Hz, 1 s) with postsynaptic depolarization to –10 mV. (Modified from Meis et al., J Physiol, 2012).
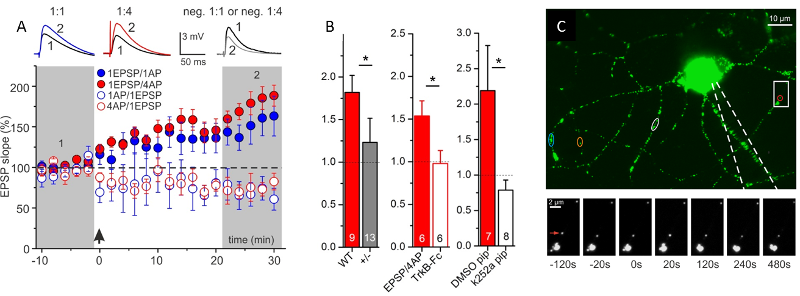
Fig.2: Secretion of endogenous BDNF from postsynaptic CA1 neurons supports t-LTP. A: Time course of t-LTP after 1:1 (1 pre- : 1 postsyn. AP) or 1:4 stimulation. STDP recordings reveal BDNF-dependent t-LTP, specifically for 1:4 pairings of pre- and postsynaptic spikes. B: Inhibition of 1:4 induced t-LTP by 3 different manipulations inhibiting BDNF/TrkB signaling (BDNF KO; TrkB-Fc; k252a in postsynaptic neuron), C: Postsynaptic BDNF secretion in response to AP firing in patch clamp recorded hippocampal neuron. Green/white spots represent BDNF-GFP vesicles. Color-coded regions indicate BDNF-GFP vesicles showing release. Boxed area is shown below at higher magnification at different time points before/after AP stimulation (at 0 s). Red arrow: BDNF-GFP vesicle showing fusion pore opening and secretion. (Modified from Edelmann et al., Neuron, 2015).
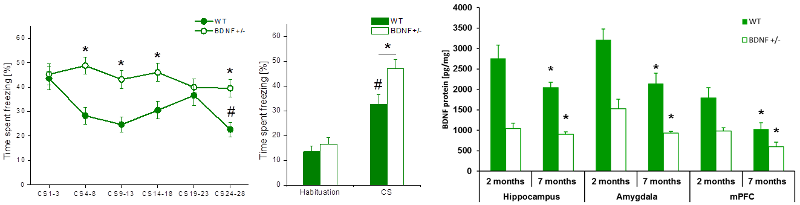
Fig.3: Fear extinction learning in 7 months old BDNF+/- and wild-type mice. Left: In contrast to 2 months old animals, 7 months old BDNF+/- mice were not able to extinguish the cued fear memory. No reduction in freezing behavior, neither during the extinction training, nor during the extinction memory test 24h later was observed. Right: Expression of BDNF-protein in brain areas known to be involved in fear extinction learning. Significant decrease of BDNF protein levels in all 3 brain areas paralleled the observed age-dependent deficit in fear extinction learning. (Modified from Psotta et al., Neurobiol Learn Mem, 2013).